wordpress-seo domain was triggered too early. This is usually an indicator for some code in the plugin or theme running too early. Translations should be loaded at the init action or later. Please see Debugging in WordPress for more information. (This message was added in version 6.7.0.) in /var/www/reachemchemicals.com/blog/wp-includes/functions.php on line 6131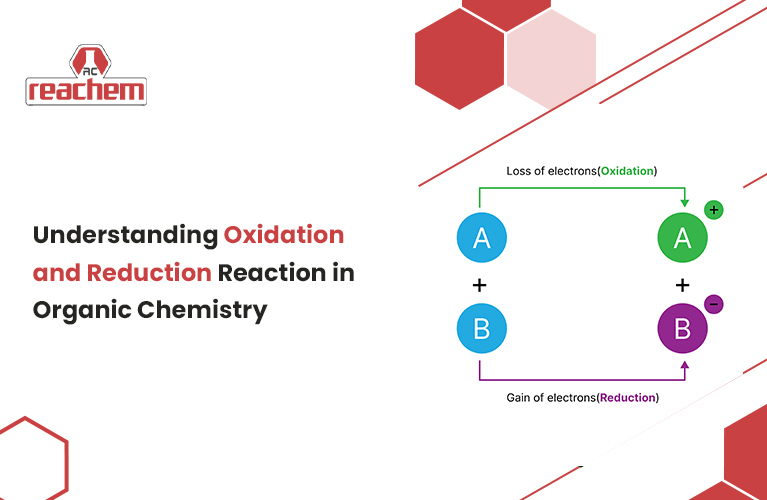
Organic chemistry, a field often overlooked, fundamentally revolves around the behaviour of molecules and their transformations. At its core, this discipline is about how molecules change from one form to another. Of all the discussed reactions, oxidation and reduction are particularly important, especially in organic chemistry. Regardless of whether you are a student, an aspiring chemist, or simply someone with a curious mind, understanding these concepts is incredibly useful. In this blog, we will make a brief comparison of oxidation and reduction reactions.
Redox reactions are known as oxidation and reduction reactions. These are processes in which electrons are transferred from one compound to another. Further, these reactions are basic to organic chemistry and are involved in most of the important biological transformations.
Oxidation: It is the process by which electrons are removed from an atom or a molecule. As electrons are taken away from a molecule, it gets increasingly oxidised.
Reduction: Also known as the reduction process in which a molecule takes a step forward in increasing its number of electrons. Positive means there are fewer electrons around the nucleus. Meanwhile, negative means that there are more electrons around the nucleus. Therefore, when a molecule gains electrons, it becomes more reduced.
In organic chemistry, these reactions may include addition or subtraction of specific oxygen or hydrogen atoms.
This is not only about the theoretical chemistry concepts like oxidation and reduction reactions but also about the reactions for the organic compounds. Such reactions are common in energy-producing pathways like the oxidation and photosynthesis processes. They are widely used in industries where they help to synthesise different chemicals and drugs.
Identifying whether a molecule is being oxidised or reduced in a reaction is crucial in understanding the process. Here are a few indicators:
In organic chemistry, these changes are often subtle and require careful analysis of the molecular structure.
Some common examples of oxidation and reduction reactions in organic chemistry to better understand how these processes work are:
Alcohols are classifications of organic compounds that can be oxidised to give out different products. The product formed depends on the kind of alcohol that is being oxidised as well as the oxidising agent that is used.
These reactions are essential in many industrial processes, such as the production of perfumes, pharmaceutical drugs and plastics.
Aldehydes and ketones are both functional groups. They undergo reduction in the presence of a reagent such as lithium aluminium hydride (LiAlH4) or sodium borohydride (NaBH4).
These reactions are prevalent in organic synthesis. Here, large, complicated molecules are built from simpler ones.
Alkenes include carbon-carbon multiple bonds and are known to undergo oxidation to produce different products. This depends on the type of oxidants used and the nature of oxidation.
These oxidation reactions are helpful in the preparation of most organic compounds, such as pharmaceuticals and polymers.
In oxidation and reduction reactions, there are defined agents that bring about these changes. Now, we will dive into the key players that drive these reactions:
Oxidising agents are those which can take electrons and, in the process, reduce the other substance. In organic chemistry, common oxidising agents include:
These agents are significant in different organic transformations, which are important in the synthesis of many molecules.
Reducing agents donate electrons and reduce another substance. Commonly used reducing agents in organic chemistry involve:
Reduction agents are very important in synthesis, whereby they are used to facilitate the reduction of a particular functional group to produce specific products.
Another principle that should also be assessed in organic chemistry is the ability to evaluate redox reactions. In this, the number of electrons that have been transferred is the same as the number of electrons that have been taken. Here’s a simple approach to balancing these reactions:
It is easier to predict what happens in a redox reaction and the chemistry going on by balancing reactions.
Oxidation and reduction reactions are more than just academic concepts. They have practical applications in various fields, which are:
These applications indicate why chemists and students ought to learn about redox reactions, as such processes form the basis of many events experienced in daily life.
Chemical reactions such as oxidation and reduction reactions help one understand oxidation numbers in organic chemistry. Therefore, it enhances understanding of chemistry. These reactions form the basis of several biochemical and industrial applications. Thus, it is of immense importance for anyone who has a passion for chemistry to understand such reactions. Regardless if used in a lab, an academic setting or in general, the rules of redox chemistry cannot be underestimated by anyone interested in the science of molecules.